There are several case reports describing a temporal correlation between the first clinical manifestation of multiple sclerosis (MS) and the occurrence of relapses with vaccination against SARS-CoV-2. Here we report a case of a 33-year-old male who developed partial right upper and lower extremities numbness 2 weeks after receiving Johnson & Johnson’s Janssen COVID-19 vaccine. The brain MRI performed during diagnostics in the Department of Neurology detected several demyelinating lesions, one with enhancement. Oligoclonal bands were present in the cerebrospinal fluid. The patient was treated with high-dose glucocorticoid therapy with improvement and the diagnosis of MS was made. It seems plausible that the vaccination revealed the underlying autoimmune condition. Cases like the one we reported here are rare, and—based on current knowledge—the benefits of vaccination against SARS-CoV-2 far outweigh the potential risks.
Introduction
Among many environmental factors contributing to the pathogenesis of multiple sclerosis (MS), it is suspected that infectious agents play a triggering role. The most often implicated are the Herpesviridae family viruses, the John Cunningham virus, and human endogenous retroviruses (1). There are several case reports describing a temporal correlation between the first clinical manifestation of MS and the occurrence of relapses with vaccination against SARS-CoV-2 (2, 3). The data are still very limited, and the cause-and-effect relationship is vague, but those cases need to be noted. Furthermore, the COVID-19 pandemic is ongoing, and such data will be crucial for future analysis.
Case report
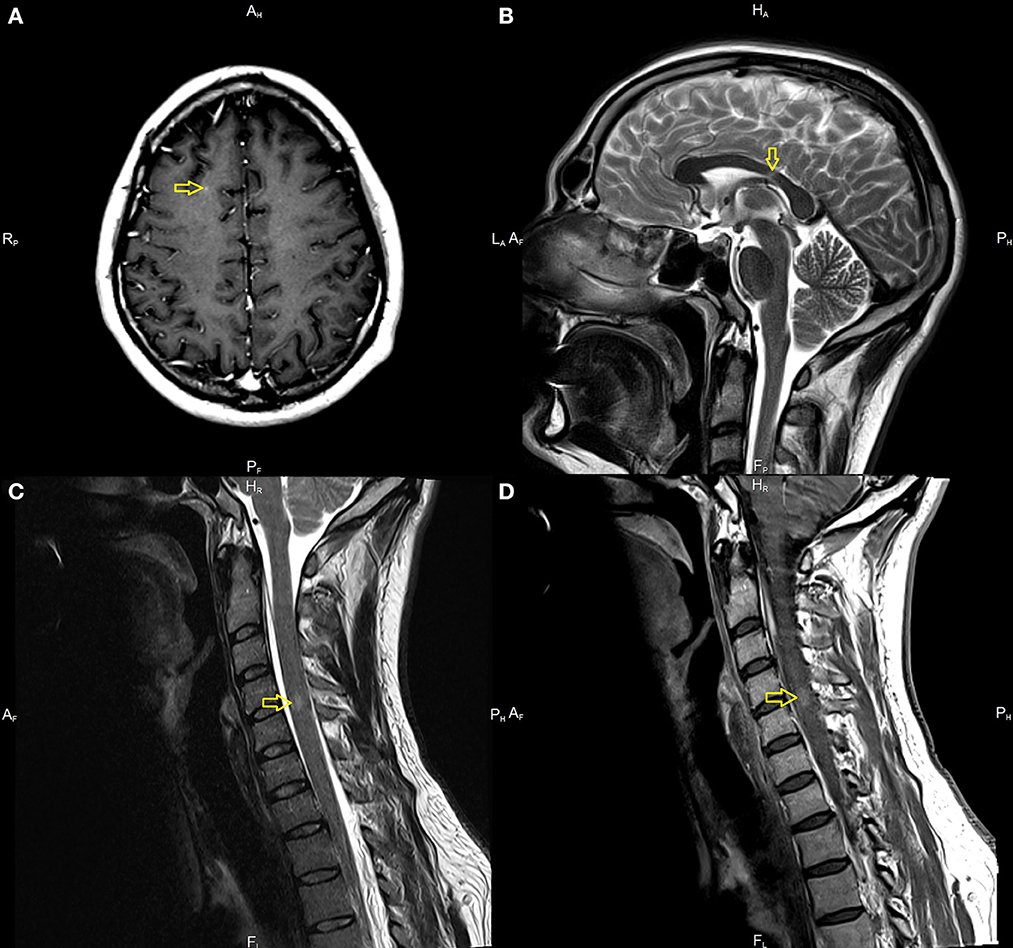
A 33-year-old male with a history of mild, well-controlled hypertension developed right upper and lower extremities numbness 2 weeks after receiving Johnson & Johnson’s Janssen COVID-19 vaccine. In the prior period, the patient was not positive for coronavirus disease 2019 (COVID-19) infection and did not present any symptoms suggesting the presence of SARS-CoV-2 infection. Due to the above symptoms, the patient was admitted to the Department of Neurology. The examination revealed right side sensory impartment (loss of pain and temperature feeling) at the C5 level. Magnetic resonance imaging (MRI) scans of the brain and thoracic and cervical spine were performed. The brain MRI detected several demyelinating lesions, one with enhancement (in the subcortical region of the right frontal lobe). The spinal cord imaging showed an enhancing lesion consistent with demyelination at the fourth/fifth cervical vertebrae (C 4/5) and one smaller lesion at the level of the third/fourth cervical vertebrae (C3), without enhancement (Figure 1). Cerebrospinal fluid (CSF) analysis demonstrated moderate pleocytosis (25 leukocytes/μl; normal range 0–5 leukocytes/μl), elevated IgG index (0.84; normal range 0.0–0.7), and the presence of oligoclonal bands (serum unmatched CSF oligoclonal bands).
Other evaluations from serum and CSF were unremarkable (serum: C-reactive protein, erythrocyte sedimentation rate, blood count were in the limits of the laboratory standard; CSF: glucose value 76 mg/dl with normal range of 50–80 mg/dl, protein value 44 mg/dl with normal range of 15–45 mg/dl). Diagnostic tests for infectious were negative. These included an examination of the presence of Borrelia burgdorferi and tick-borne encephalitis virus; the region of the patient living is endemic for both. Tests for the presence of anti-aquaporin-4 antibodies, myelin basic protein antibodies, and antibodies to myelin oligodendrocyte glycoprotein were negative. The evaluation for other autoimmune diseases was negative and included: rheumatoid factor, antiprothrombin, antinuclear, antiphospholipid, and antineutrophil cytoplasmic antibodies, antigens against :dsDNA, nucleosome, histosome, SS-A, Ro-52, SS-B, nRNP/Sm, Sm, Mi-2alfa, Mi-2beta, Ku, A, and B centromeres, Sp100, PML, PM-Scl100, PM-Scl75, Scl-70, RP155, gp210, PCNA, and DFS70.
Source: FrontierSin